Photo by rawpixel on Unsplash
Learn more about large-document management systems and the evolution of the industry in the first part of this two-part series.
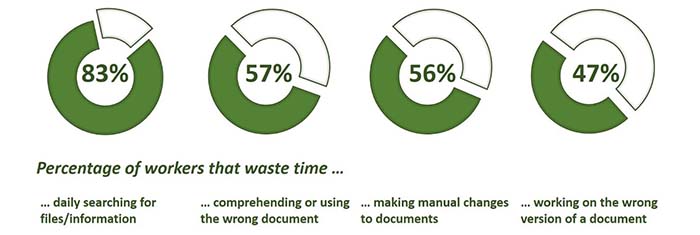
Companies operating in highly-regulated environments produce a lot of documentation. The systems that evolve to handle document management usually combine labor intensive, inefficient manual processes with some level of technological automation. In fact, a 2012 study by Harris Interactive found significant impacts on worker productivity due to inefficient document management.
 When a company decides to evaluate more comprehensive document management solutions, it can be difficult to know where to begin. In this article, Alexandre Loukakos, CEO of DocTech, provides advice on how to proceed with the task of implementing a large document management solution when operating in a highly regulated industry.
When a company decides to evaluate more comprehensive document management solutions, it can be difficult to know where to begin. In this article, Alexandre Loukakos, CEO of DocTech, provides advice on how to proceed with the task of implementing a large document management solution when operating in a highly regulated industry.
Alexandre recommends doing a phased approach with extensive analysis at each phase to ensure business needs and quality requirements align with industry standards and usability.
Identify the Current State
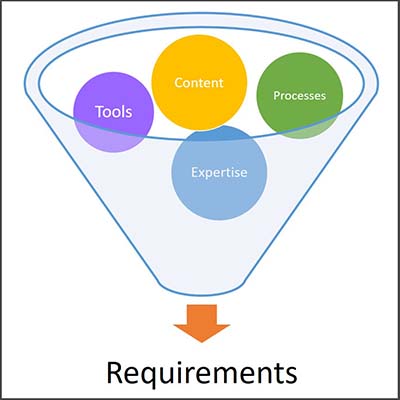 Before committing to spending huge sums on an enterprise-level document management solution, Alexandre advises that companies should do a very thorough assessment of current systems and work processes by asking a lot of questions and analyzing all aspects of document maintenance as they currently exist:
Before committing to spending huge sums on an enterprise-level document management solution, Alexandre advises that companies should do a very thorough assessment of current systems and work processes by asking a lot of questions and analyzing all aspects of document maintenance as they currently exist:
- How much documentation currently exists? How is it categorized and organized?
- How is the document life cycle workflow organized, and what tools are used at each phase?
- What documents are required for regulatory compliance? What is required for industry standards compliance or certification? How many existing documents are repurposed for multiple compliance needs?
- Where is documentation stored? Who are the designated owners of documentation?
- How much of documentation content is duplicated? How much is obsolete?
- How do existing tools and processes flag content obsolescence and provide updates?
- What is the average number of revisions required to produce an updated document?
- How is the content (text and visuals) sourced?
- What content outputs are required? How many languages?
- How many steps does it take to produce a new document? To revise an existing document?
- What tools are currently used at each step? (email, word processing, tracking, graphics, repositories, etc.) Are these tools up-to-date, proprietary, process-driven or data-driven?
- How many roles/teams are required to create and produce documentation? What other stakeholders need to be considered?
- What internal review and approval processes exist and how are they executed?
- What is the lifecycle for document maintenance? What are the internal and external audit and reporting requirements?
- How are errors tracked and mediated?
- How do auditors review current documentation?
- What are users (those involved in document creation and management) biggest pain points?
Alexandre says that one of the biggest, costliest and most frequently occurring mistakes a company can make is “to try to impose or force a system (commercial or in house) without considering the end-user feedback. This alone in itself is responsible for more than 70% of software implementation failures in the aeronautic industry.” And by not thoroughly analyzing user needs, as well as compliance and regulatory compliance requirements, companies risk ending up with an “unhealthy hybrid system: old plus new features and processes, or worse, random old plus random new ones.”
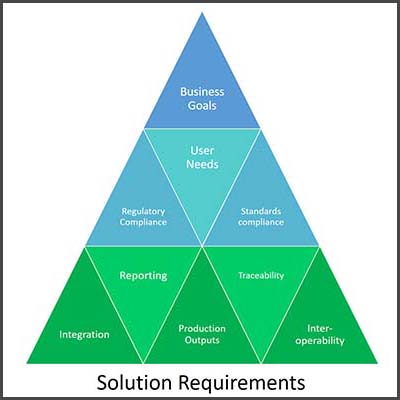 Create Comprehensive Requirements
Create Comprehensive Requirements
An in-depth assessment of the current document management ecosystem makes it easier for companies to identify what they really need, versus what might address one pain point while negatively impacting other areas. Alexandre suggests considering these requirements from both the operational and quality perspectives.
Systems such as DocTech aim to maximize flexibility so that companies can address their own specific operational goals and constraints and fully integrate quality system requirements or standards imposed by regulatory or by the organization itself. For example:
- Audit reporting: Audit report requirements can vary widely, and the system should be able to track both the actual number and content of changes from one version to another. For example, 8000-page document may only have 20 pages of changes in a given report if the changes are mostly to visuals. In addition, the system report may show a small number of total changes, if many of the approval/review processes are automated within the system.
- Ad hoc reporting: Reporting needs change over time and between scenarios, so a strong solution allows for ad hoc reporting that can be maintained in the background to reduce distractions for users and to promote greater efficiency.
- Multiple language management: a comprehensive document management solution should handle multiple languages at the application level and at the content level for maximum collaborative efficiency. For instance, In DocTech, a Chinese collaborator can use the Chinese interface for convenience to work on a document to be produced in English. At the same time, a collaborator in Europe can contribute to the creation of a document in Chinese, using the application interface language of its own choice.
- Tracking and logging: Automatic logging and comparison of content changes provides auditors with a quick and complete summary of the deviations from the standards. With DocTech, auditors can manage the summary with a dynamic table of contents to eliminate manually reviewing thousands of pages. Automatic logging also improves traceability, a critical need for auditors.
- Multiple outputs: PDF remains one of the primary outputs of document management systems in highly regulated environments. A good solution produces PDF, but also supports other outputs such as HTML and XML that can integrate with other systems
- Role management: A comprehensive document management system needs to ensure security of the content and the regulatory process and reporting by ensuring that access is managed by role, including content creators, auditors (both internal and external), reviews, approvers, and production managers.
Above all, Alexandre says, companies looking to implement the right large document management solution need to take a thorough “try before you buy” approach and ask as many questions as possible throughout.
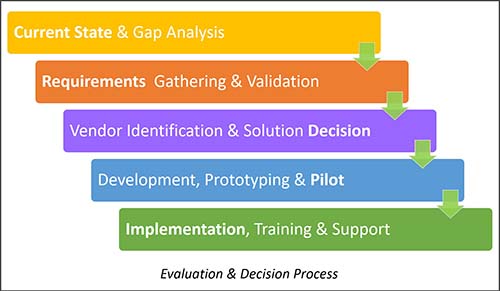 Evaluation and Decision
Evaluation and Decision
During vendor selection, companies should review results of actual document production and report processes. They also need to set up at least one test phase with two-to-three users in the content creation roles and one user in a quality control role. The test group should be able to recreate the results provided in the sample documents and reports. “The overall test approach provides invaluable knowledge about what you do want and don’t want in your system,” Alexandre notes.
Hiring consultants to assist with selection and implementation is not an absolute requirement, and Alexandre says that consultants can both simplify and complicate the process. All information on standards are widely available from their respective regulatory bodies, and a consultant can save a great deal of time in reviewing and understanding the “sometimes colossal” standards documentation.
On the other hand, consultants can force companies to rethink their assumptions about their documentation processes, which, although time consuming, often prove to be time well spent.
Summary
Organizations in highly regulated industries face some daunting challenges in managing their very large documentation sets. Whether choosing to build from the ground up, or integrate and modernize existing tools, take a methodical approach to analyzing what you have now, what you’ll need in the near future, and all the pain points and road blocks in your current processes. Then you can make the right decision on deploying the right solution to meet those challenges.
You may also like:
-
Challenges of Large-Document Management in Highly Regulated Environments
- A Simpler Solution for Content-intensive Documentation: Profile of DocTech